 We are often asked about the differences between the various ethylene-propylene rubber compounds. There are a slew of acronyms used for EP and there are also different cure systems. What are the differences? Well, it is important to know what the differences are because what may work well in one application, may not work well in another.
We are often asked about the differences between the various ethylene-propylene rubber compounds. There are a slew of acronyms used for EP and there are also different cure systems. What are the differences? Well, it is important to know what the differences are because what may work well in one application, may not work well in another.
Let’s start by giving a general overview of ethylene-propylene rubber. EP has excellent ozone, UV, and weather resistance. It also has good mechanical properties. In regards to fluids, EP’s typically have resistance to dilute acids, alkalis, ketones, alcohol, automotive brake fluids, phosphate ester-based hydraulic fluids and water. On the flip side, EP’s will swell considerably in petroleum based fluids.
Ethylene-propylene elastomers can be compounded to operate in continuous service temperatures of
-65°F to +300°F. In steam service the EP can operate at up to 400°F.
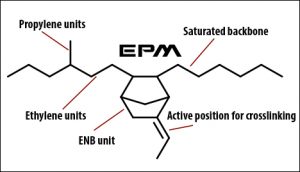
An EP molecule is made up of smaller molecules call monomers. The monomers are linked together to form a chain. The types of monomers used, dictate the type of EP you have.
Let’s define the most common acronyms you see for EP: EPM, EPDM EPT, and EPR.
EPM is what you call the ethylene-propylene copolymer. The copolymer is just what it sounds like; it is made up of two monomers, ethylene and propylene.
EPDM is the acronym for ethylene-propylene-diene monomer. This type of EP is made up of 3 monomers. When you have 3 monomers it is called a terpolymer, thus EPT stands for ethylene-propylene-terpolymer and is the same thing as EPDM.
EPR simply stands for ethylene-propylene rubber and can be used to describe the entire family of ethylene-propylene elastomers.
When EP was first invented the only available cure system utilized peroxide. Peroxide cured materials are difficult to process because they stick to the mold, they are hard to deflash, and are susceptible to scorching. Posed with this problem, rubber chemists added the 3rd monomer, the diene monomer, to the EP molecule. This 3rd monomer allowed for a cure site for the sulfur cure system.
Sulfur cured EPDM elastomers are easier to process and generally cost less than the peroxide cured materials. You would generally use sulfur cured EP products for environmental seals where service temperatures do not exceed 212°F. This may be window glazing, potable water applications, or where resistance to weather is important.
You would use the peroxide cured EP’s for more demanding applications where temps may reach 300°F. The peroxide cured EP’s offer better heat resistance, and compression set resistance. Typical applications are automotive brake systems or steam applications.
If you have any questions, contact us or visit our website.
